Learning Outcomes
i. Students will be able to sketch a Daniell cell.
ii. Students will be able to label the cathode, the anode, and the direction of electron flow in a Daniell cell.
iii. Students will be able to understand the setup of a voltaic cell.
Introduction
A voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. This is accomplished through a process called redox reaction, in which one species loses electrons (oxidation) and another species gains electrons (reduction). The electrons that are lost are transferred to an external circuit, where they can do work.
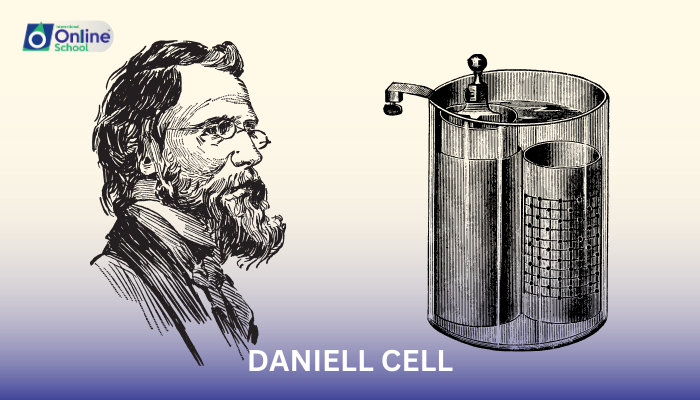
The Daniell cell is a type of voltaic cell that was first invented by John Frederic Daniell in 1836. It is a simple cell that consists of two electrodes: a zinc anode and a copper cathode. The electrodes are immersed in separate solutions: the zinc anode is in a solution of zinc sulfate (ZnSO4), and the copper cathode is in a solution of copper sulfate (CuSO4).
To sketch a Daniell cell, follow these steps:
- Draw two circles, one for the zinc anode and one for the copper cathode.
- Label the zinc anode Zn and the copper cathode Cu.
- Draw a salt bridge between the two solutions. The salt bridge is a tube that allows ions to flow between the two solutions without mixing them.
- Draw arrows to show the direction of electron flow. Electrons flow from the zinc anode to the copper cathode through the external circuit.
- Label the cathode as the positive electrode (+) and the anode as the negative electrode (-).
The Daniell cell works because zinc is more reactive than copper. This means that zinc will lose electrons more easily than copper. When the zinc anode loses electrons, it becomes zinc ions (Zn2+). These zinc ions dissolve into the zinc sulfate solution. At the same time, copper ions (Cu2+) from the copper sulfate solution gain electrons and become copper atoms. These copper atoms deposit onto the copper cathode. The electrons that flow from the zinc anode to the copper cathode pass through the external circuit, where they can do work.
The Daniell cell is a simple voltaic cell that can be used to generate electricity. It is a good example of how redox reactions can be used to convert chemical energy into electrical energy.
Additional Examples
- The lead-acid battery is a type of voltaic cell that is commonly used in cars.
- The nickel-cadmium battery is a type of voltaic cell that is commonly used in portable electronic devices.
- The fuel cell is a type of voltaic cell that uses hydrogen and oxygen to generate electricity.